The BG5 Biofilm Growth Detector system, originally developed for and proven in power plant cooling…
Biofilms? MIC? What Are They?
Has the heat conversion efficiency of your heat exchangers degraded? Is the flow of your cooling water system being impeded? Are you repairing or replacing equipment due to localized corrosion causing through-wall failure? Inefficiencies and equipment failures are big problems in any industrial process, but the cause of the problem may be smaller than you think. You might have a biofilm problem. Bacteria floating in a cooling or process water can become colonies on wetted surfaces and can form robust biofilms over remarkably short times. Biofilms are collections of living and dead cells that are enclosed in an extracellular polymeric substance matrix secreted by living organisms. The unchecked growth of biofilms can significantly decrease thermal efficiency on surfaces as the biofilm acts as an insulating layer. Highly localized chemical effects can also be created that lead to microbiologically influenced corrosion (MIC).
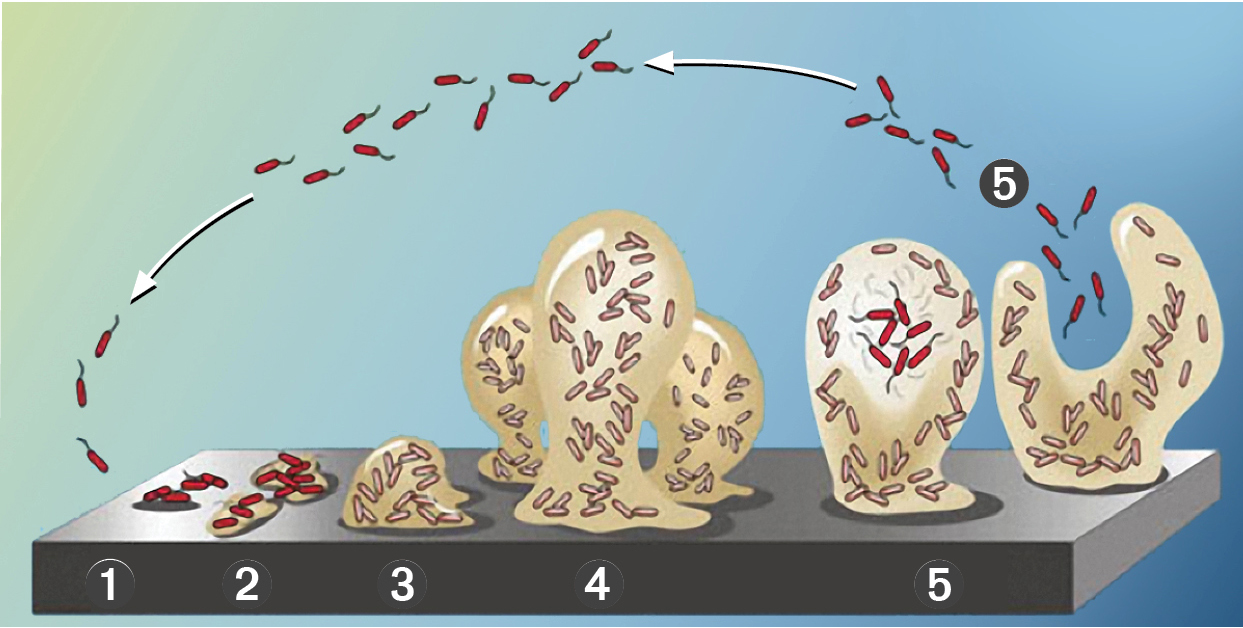
But how do biofilms even develop? When the minimal conditions for bacterial growth are met, (temperature, nutrients, oxygen content) a biofilm begins development as bacteria near a surface consume nutrients and multiply. The bacteria become sessile, affixed to a surface, by secreting exopolymeric saccharides (EPS) that allow the cells to adhere to the surface and other cells. Growth continues and other bacteria species join the colony as all these cells use quorum sensing to perceive and respond to microbial population density. Aerobic (with oxygen) and anaerobic (without oxygen) regions form within the biofilm, supporting a diverse and synergistic community of microbes. The biofilm grows, building mass and thickness to a point of reaching equilibrium where, as more growth occurs, a natural erosion of viable bacteria from the outer surface of the biofilm also occurs, releasing living cells back to the bulk fluid that can seed growth on other surfaces of the system.
Well so what? Now I have a biofilm. Why does that impact my performance? Bacteria and biofilms are mostly water. Therefore, the thermal conductivity of biofilm is comparable with that of water, however, biofilms are fixed to the surface and will act as stagnant insulating layers hindering the heat transfer across surfaces. As the biofilm grows, it also traps ions and creates localized chemical and physical gradients at the metal surface allowing corrosion mechanisms for microbiologically influenced corrosion (MIC) to take place.

The presence of a biofilm is necessary for MIC to initiate, but not necessarily required for MIC to propagate. There are conditions where localized corrosion may be initiated by MIC but propagate completely abiotically [2]. One way to completely mitigate the risk of MIC occurring is to have a completely sterile system that is free from any living bacteria. However, doing so is immensely impractical in real world scenarios. The addition of chemical biocides and dispersants help to slow or eliminate the buildup of bacteria and biofilms on system surfaces. However, the overuse of these chemicals can increase the oxidizing power of the environment and aggravate an already existing corrosion condition that may have been initiated by MIC attack or not.
SI offers a solution to monitor the effectiveness of these chemical treatments. The BIoGEORGETM BG4 Biofilm Growth Detector system provides biofilm activity data online and in real-time so facilities can better correlate biocide chemical use with biofilm growth decreases. Monitoring biofilm activity in this way helps avoid overdosing and underdosing biocide. For more information visit biofilmgrowth.com.
Edward Dougherty – edougherty@structint.com
References
[1] J. W. Costerton, G. G. Geesey and P. A. Jones, “Bacterial Biofilms in Relation to Internal Corrosion Monitoring and Biocides”. Corrosion 87 paper No. 57 SanFransisco, CA. 1987 [2] Licina, George J. “Treatment Optimization Through Effectiveness Monitoring”. NACE Corrosion 2013 Conference and